Abstract
Introduction
Chimeric antigen receptor (CAR)-T cell therapy is considered a therapeutic breakthrough in cancer immunotherapy especially targeting subsets of B cell leukemia and lymphomas. The barriers still limiting the clinical response to CAR-T cell therapy range from antigen escape, impaired tumor infiltration, severe toxicities, insufficient anti-tumoral effects, to CAR-T cell exhaustion. Varying approaches are underway to translate CAR-T cell therapy into the treatment of solid tumors and further hematological malignancies.
Methods
Based on literature review and single-cell analysis of chromatin structure, we identified potential genetic drivers of CAR-T cell exhaustion and insufficient anti-tumoral effects. To investigate the underlying cellular and genomic mechanisms, we established polyclonal gene knockouts utilizing CRISPR/Cas9 ribonucleoprotein (RNP) based genome editing. Knockout efficacy was validated on a genomic level using interference of CRISPR editing (ICE)-sequencing. To determine the killing capacity of modified anti-CD19-CD28ζ CAR-T cells of luciferase transgenic CD19+ Nalm6 ALL blasts, in vitro bioluminescence-based cytotoxicity assays were performed. Repetitive killing capacity was evaluated in vitro by co-culturing modified CAR-T cells with Nalm6 cells at a 1:1 E:T ratio over 48 hours followed by another co-culture with tumor cells at a 1:1 E:T ratio over 24 hours. Continuous stimulation of the T cell receptor (TCR) was assessed by stimulating 0.2x105 modified CAR-T cells with 2 µL anti-CD3/anti-CD28 antibodies over 48 hours. Expression of extracellular and transcriptional (exhaustion) markers were assessed by flow cytometry. To elucidate the underlying functional mechanisms, we performed single-cell RNA sequencing.
Results
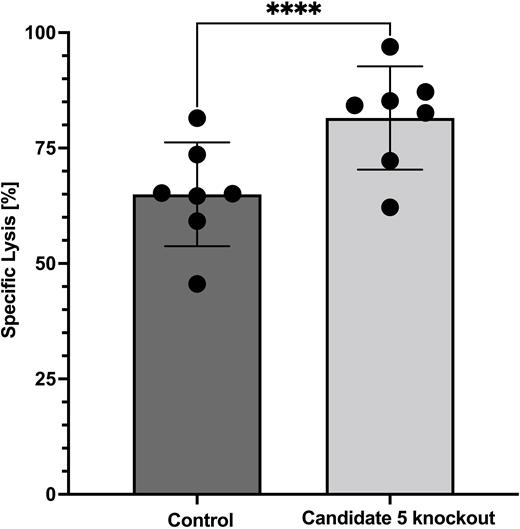
Mean knockout efficacy across our candidate genes was >90%. Cell viability after expansion over 7 and 14 days was comparable between knockout CAR-T cells and unmodified control CAR-T cells (n≥4). The proliferative capacity of CAR-T knockout cells was comparable to control CAR-T cells (n≥4). Knockout of candidate 5 gene showed significantly enhanced killing capacity in cytotoxicity assays (n≥4; p= 0.0003) (Figure 1), as well as significant downregulation of the exhaustion markers programmed cell death protein-1 (PD-1) (n≥4; p= 0.037) and lymphocyte-activation gene-3 (LAG-3) (n≥4; p= 0.028) in flow cytometry after cytotoxicity assays. Levels of cytotoxic T cell mediators Granzyme B (n≥4; p= 0.489) and Interferon-gamma (IFNy) (n≥4; p= 0.361) were not reduced compared to control group. Except for PD-1 expression (p= 0.147), these findings were reproduceable when testing the repetitive killing capacity of candidate 5 knockout CAR-T cells (n= 3). Following continuous TCR stimulation PD-1 (n≥4; p= 0.013) and LAG-3 (n≥4; p= 0.031) were significantly downregulated compared to control CAR-T cells. Levels Granzyme B (n≥4; p= 0.637) and IFNy (n≥4; p= 0.501) were found to remain stable.
Conclusions
In summary, we present a novel promising target gene to enhance CAR-T cell efficacy with high potential for clinical translation, which is currently analyzed in vivo.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal